Figure 2 from In situ EELS study of dehydration of Al(OH)₃ by electron beam irradiation. | Semantic Scholar

0.078 g Al(OH)(3). is dehydrated to Al(2)O(3). The Al(2)O(3) so obtained reacted with 6 milli-equivalent of HCl. The equivalent of AlCl(3) produced during the reaction are:

Preparation of hexagonal micro-sized α-Al2O3 platelets from a milled Al(OH)3 precursor with NH4F and NH4Cl additives
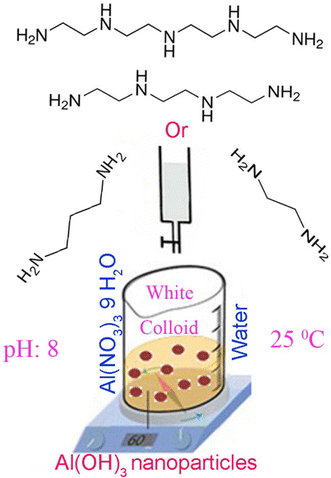
Synthesis and Characterization of Al(OH)3, Al2O3 Nanoparticles and Polymeric Nanocomposites | SpringerLink

High-Pressure δ-Al(OH)3 and δ-AlOOH Phases and Isostructural Hydroxides/Oxyhydroxides: New Structural Insights from High-Resolution 1H and 27Al NMR | The Journal of Physical Chemistry B
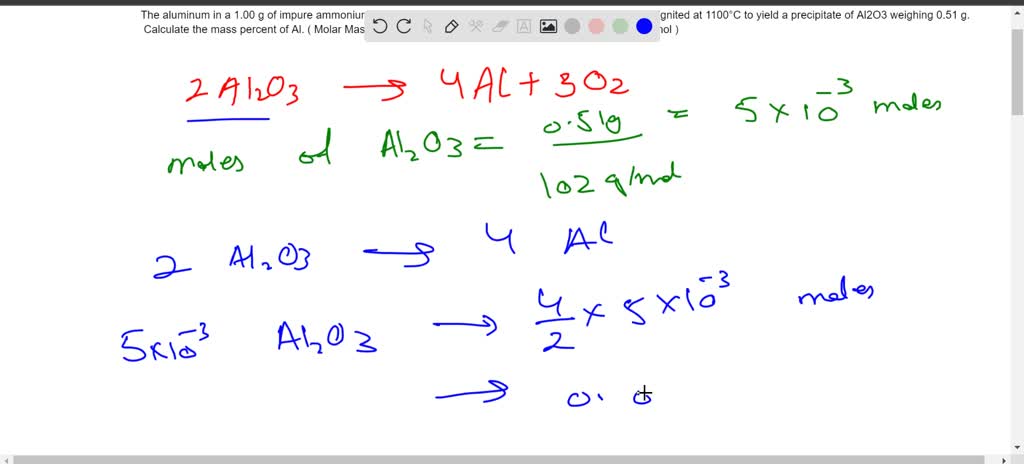
SOLVED: The aluminum in a 1.00 g of impure ammonium aluminum sulfate sample was precipitated as Al(OH)3 and ignited at 1100°C to yield a precipitate of Al2O3 weighing 0.51 g. Calculate the

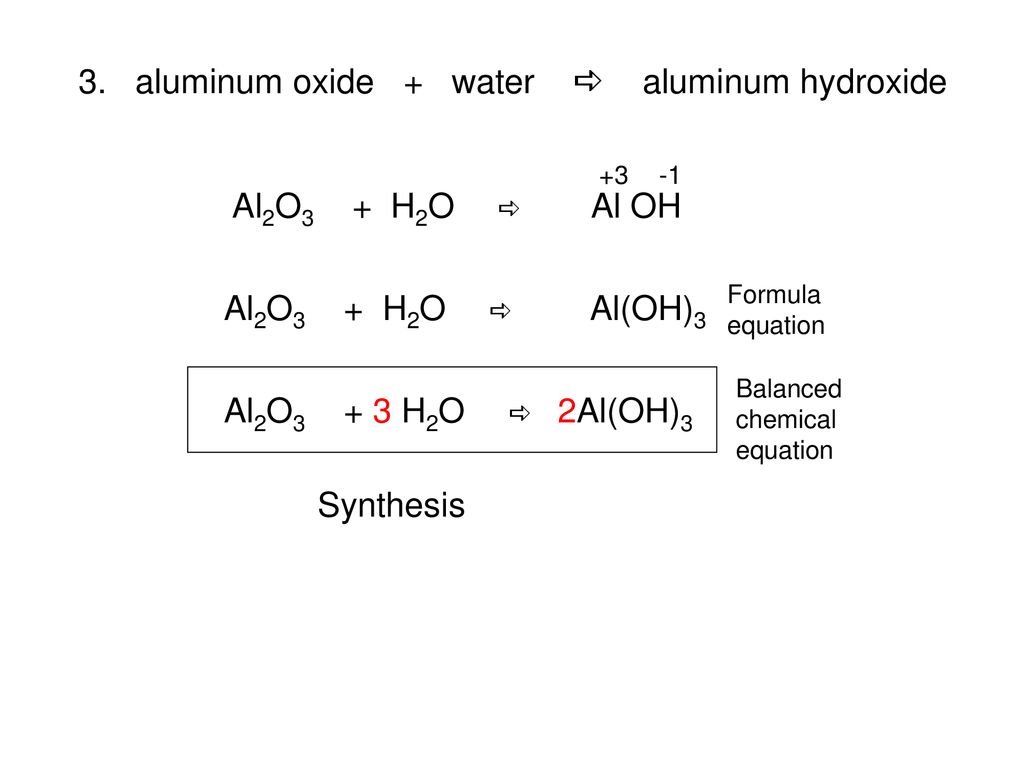
How to Balance Al(OH)3= Al2O3+ H2O|Chemical equation Al(OH)3=Al2O3+H2O|Al(OH )3=Al2O3+H2O Balance - YouTube