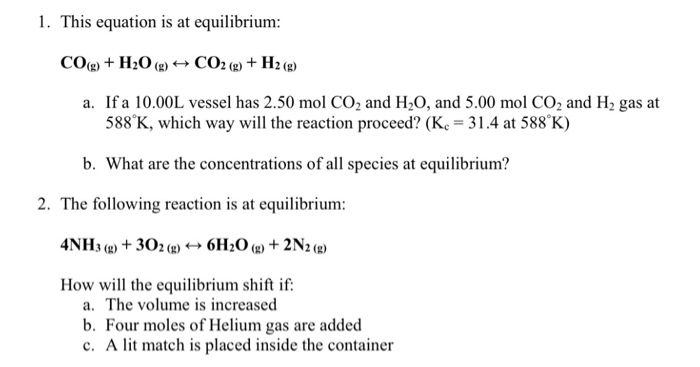
Practice How many grams of H2O are produced by completely reacting 24 g of O2? 2 H2 + O2 2 H2O convert to moles convert to the desired molecule convert. - ppt download
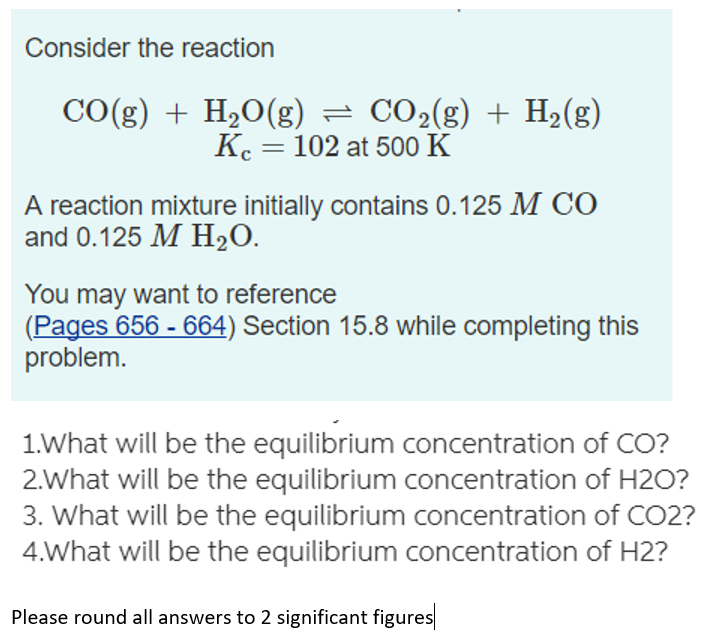
SOLVED: Consider the reaction below. H2O (g) + CH4 (g) <—> CO (g) + 3H2 (g) Kc = 4.7 at 1400 K What is Kp for this reaction at 1400 K? 6.2 x 104 4.7 8.2 x 10^8
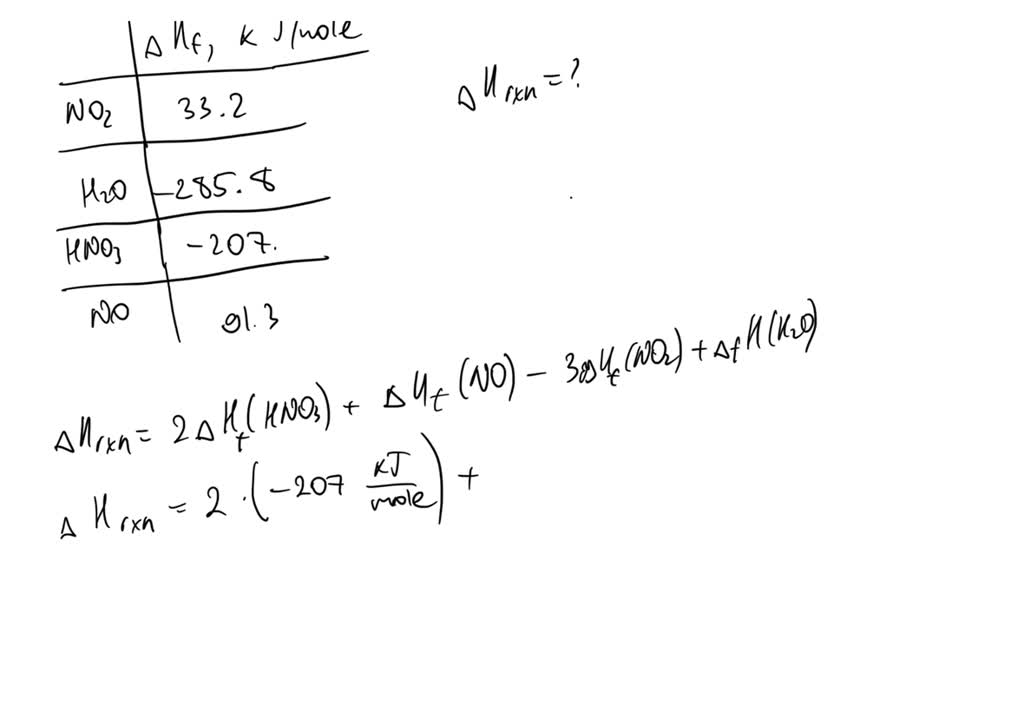
SOLVED: 4.) Consider the following reaction: 3NO2 (g) + H2O (l) ———–> 2HNO3 (aq) + NO (g) given the standard enthalpy of formations: NO2 (g) = 33.2 kJ/mole , H2O (l) = -

See: Calculate the amount of heat released when 27.0 g H2O is cooled from a liquid at 314 K to a solid at - Brainly.com
0.262g of a subs†an ce gave on combustion 0.361g of co2 and 0.14g H2O what the emperical formla of substance