![SOLVED: Which of the statements outlined below is NOI correct regarding the relationship between the hydronium and hydroxide ion concentrations, [H3O*] and [OH ], respectively, in relation to the pH of an SOLVED: Which of the statements outlined below is NOI correct regarding the relationship between the hydronium and hydroxide ion concentrations, [H3O*] and [OH ], respectively, in relation to the pH of an](https://cdn.numerade.com/ask_images/2c1d38b061434304a23e9db2b01d94f0.jpg)
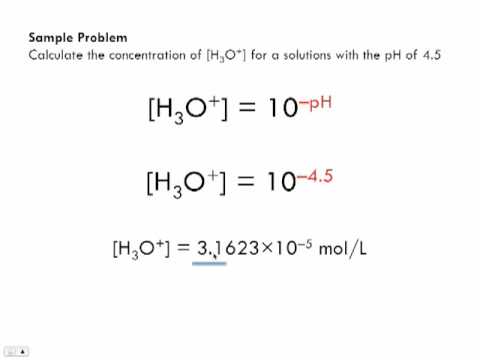
SOLVED: Which of the statements outlined below is NOI correct regarding the relationship between the hydronium and hydroxide ion concentrations, [H3O*] and [OH ], respectively, in relation to the pH of an

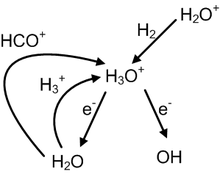
Assertion: Ammonia shows a trigonal pyramidal molecular structure.Reason: In the structure of ammonia, three atoms are attached to the central atom and thus, shows tetrahedral electron pair geometry.




![Hydronium -[H3O]+ Hydronium -[H3O]+](http://www.chemtube3d.com/images/gallery/PNGfiles%20structures/I604ST01.png)