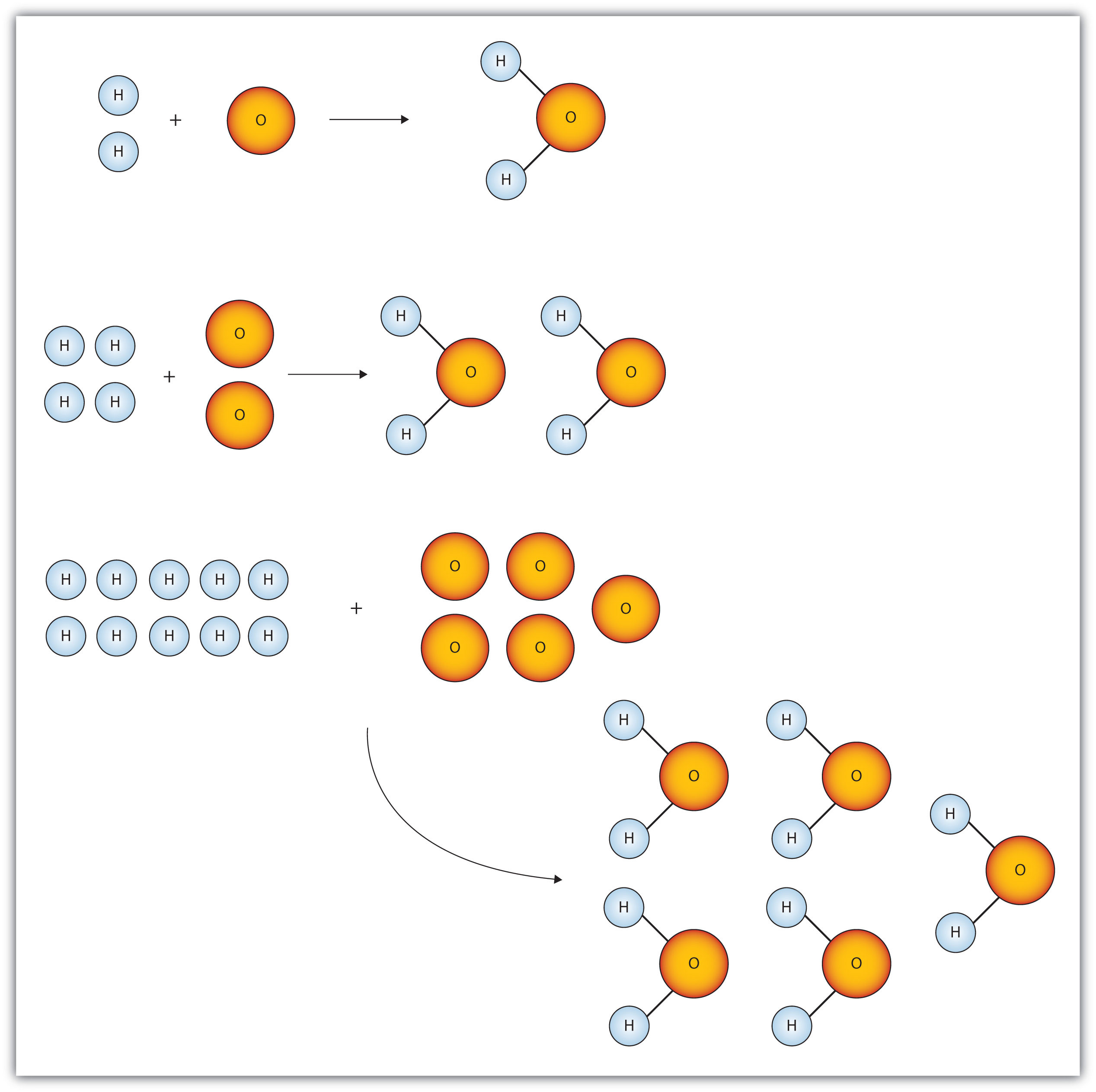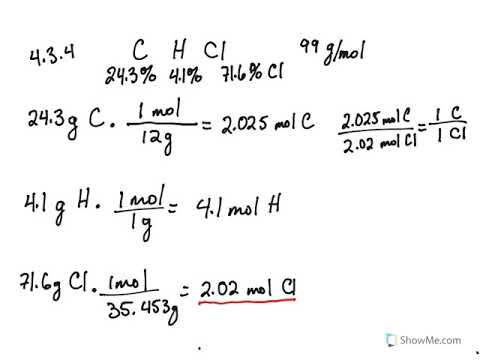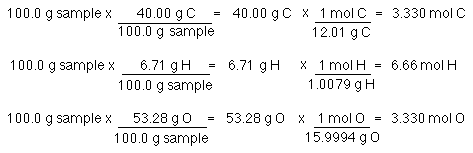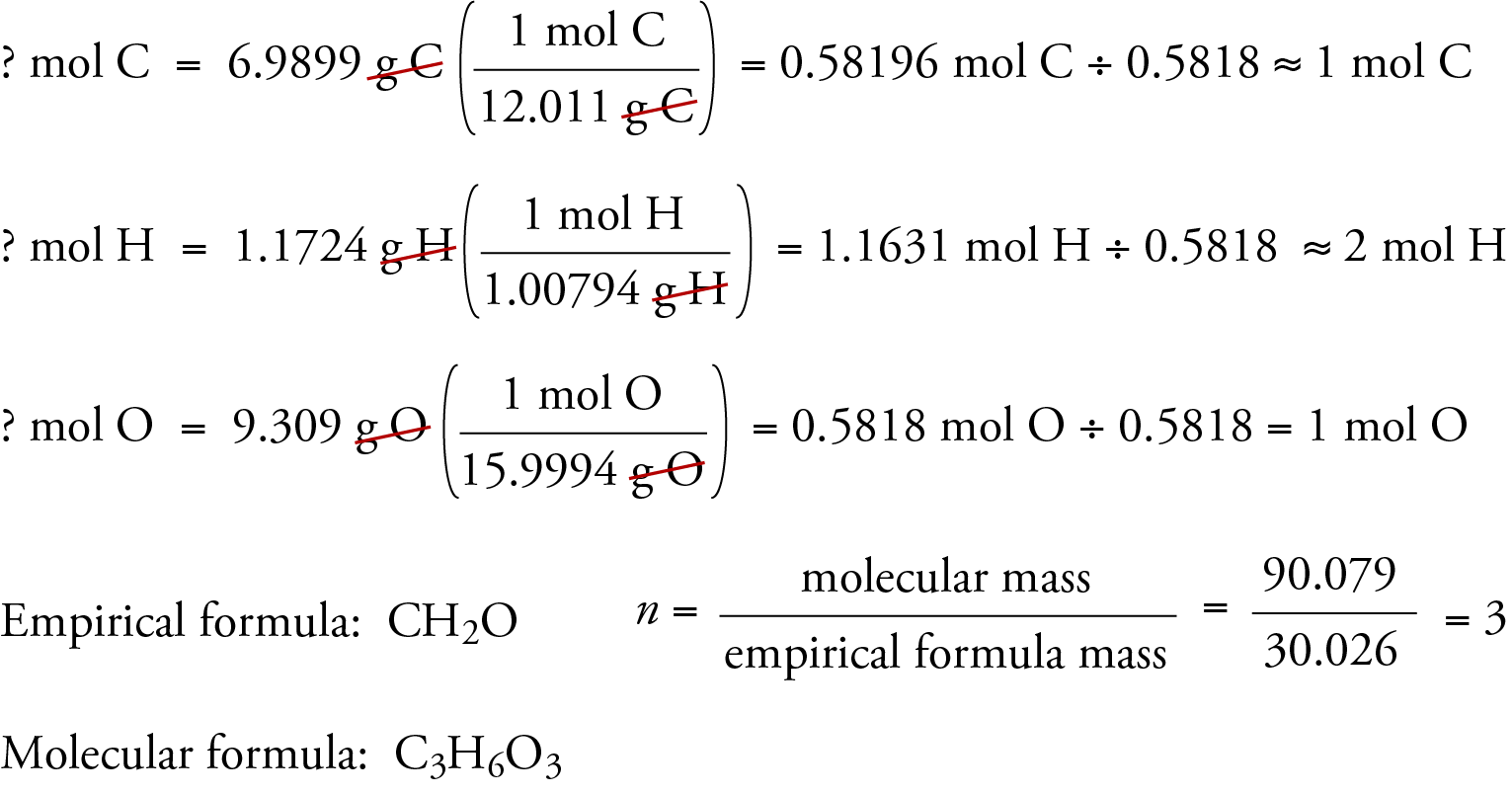
Empirical and molecular formulas for compounds that contain only carbon and hydrogen (C a H b ) or carbon, hydrogen, and oxygen (C a H b O c ) can be determined with a process called combustion analysis. The steps for this procedure are

STOICHIOMETRY CALCULATIONS COACH COX. MOLE TO MOLE CONVERSIONS Converting from moles of one substance in a chemical reaction to moles of another substance. - ppt download